3dpunto
nice


























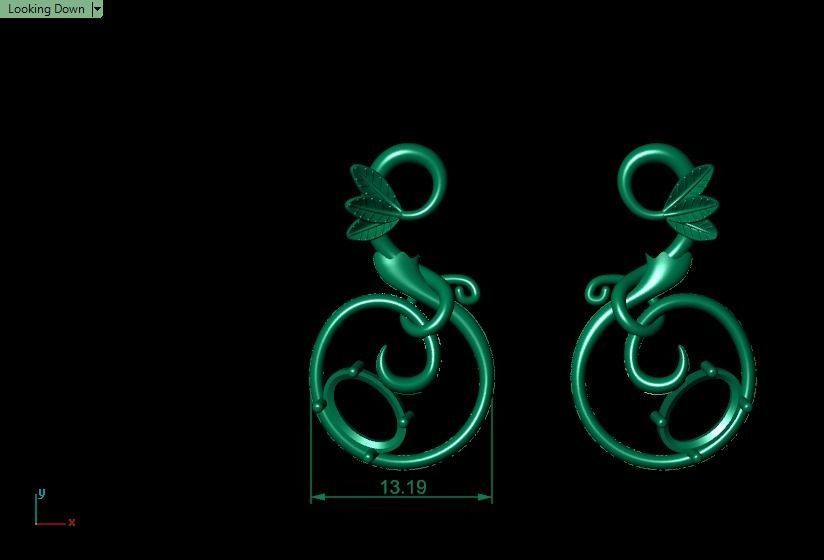
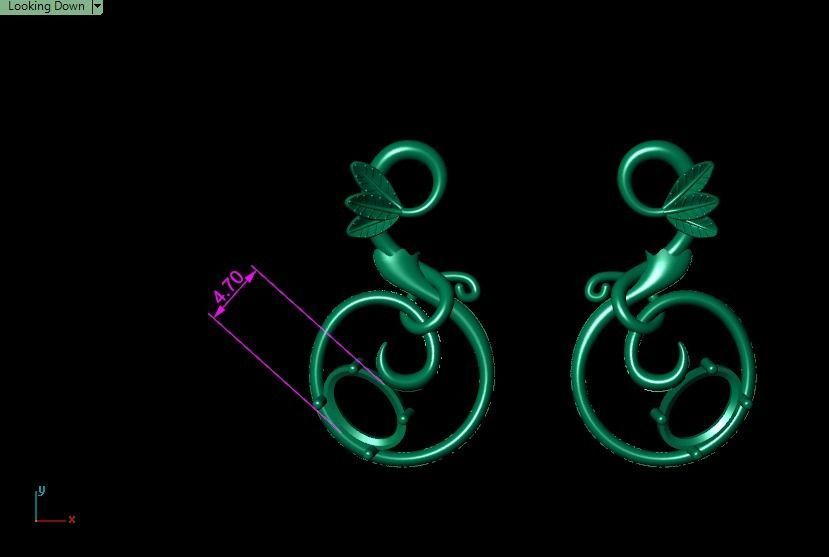
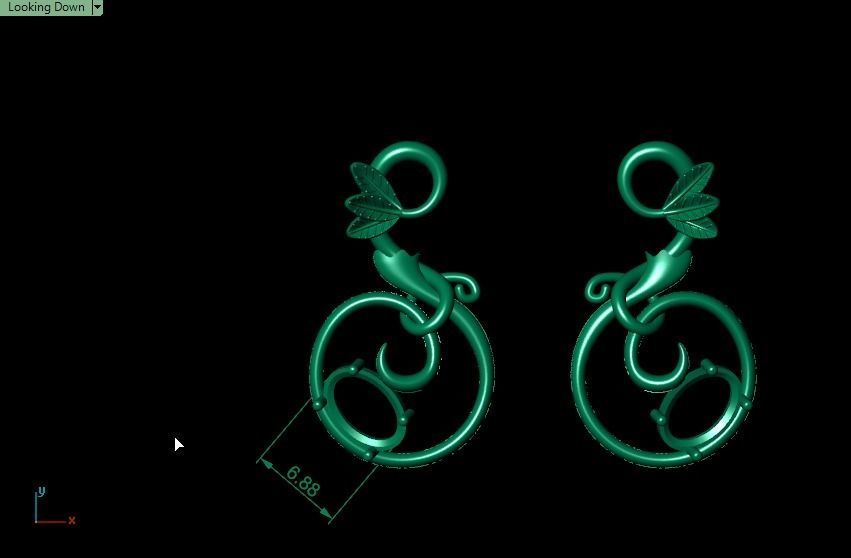

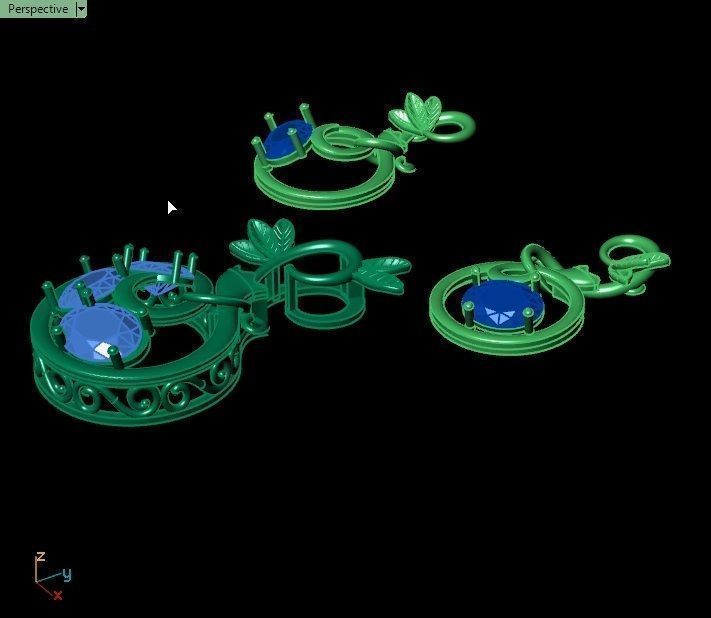
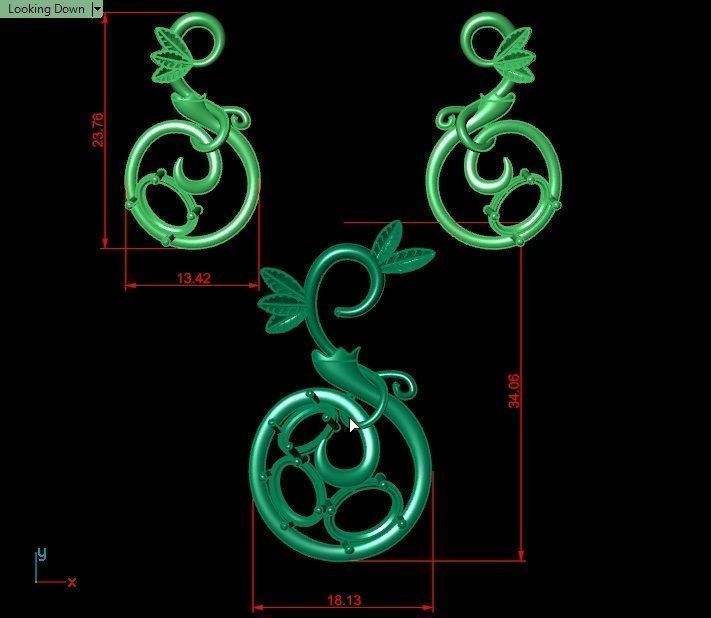
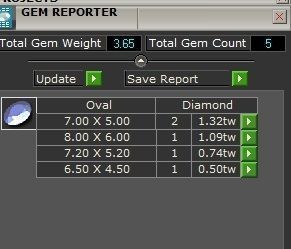
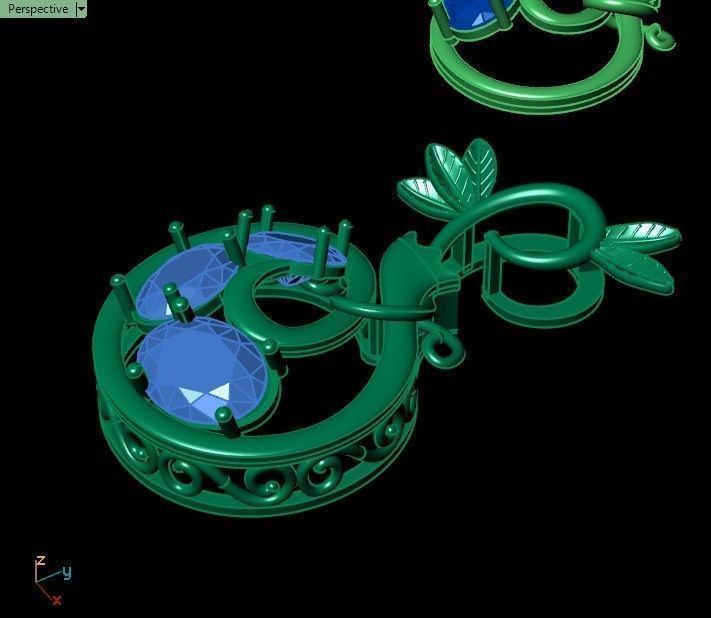
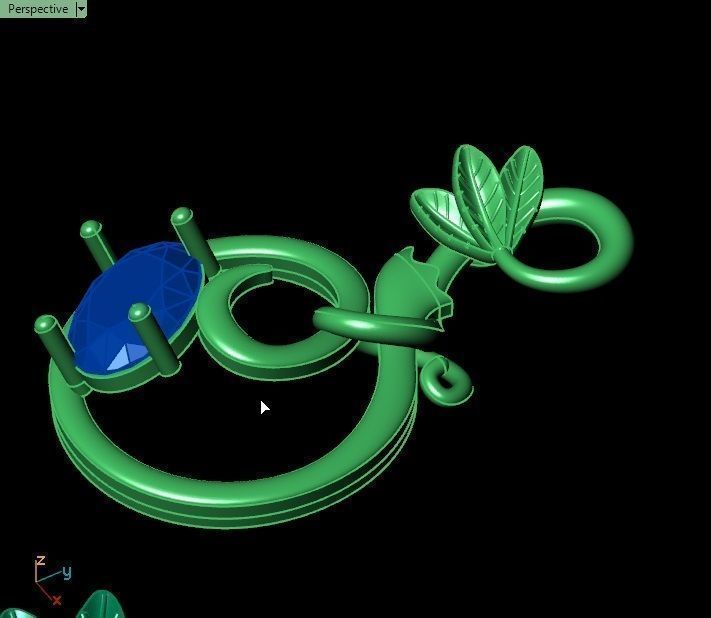



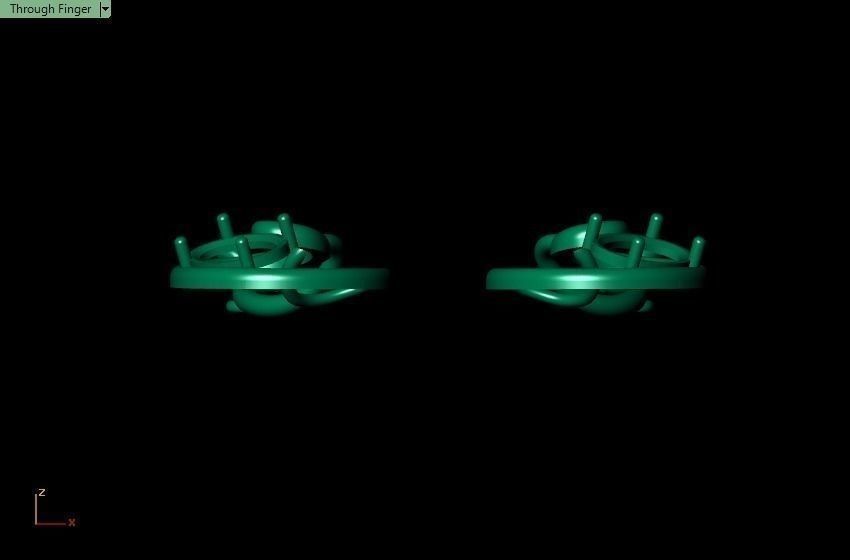




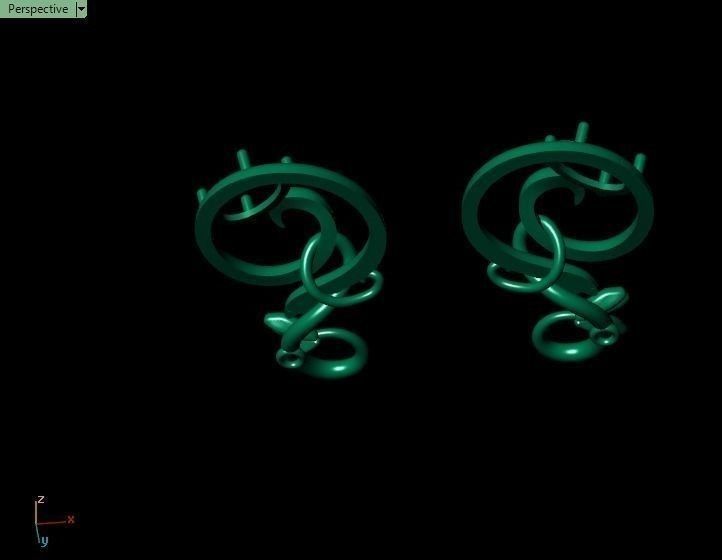















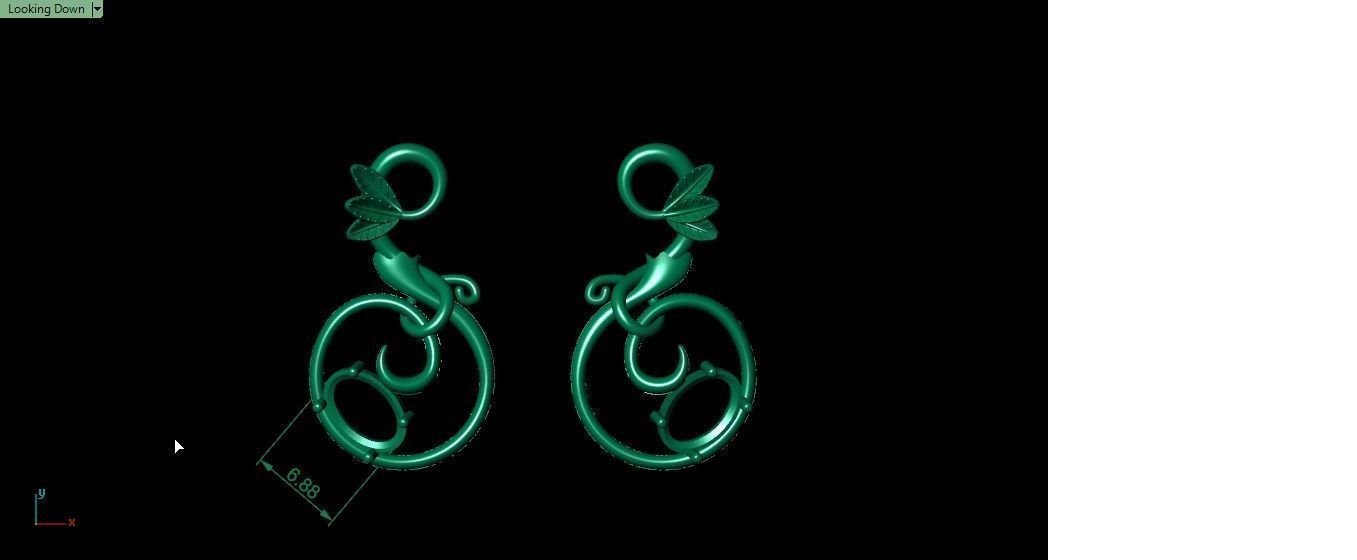

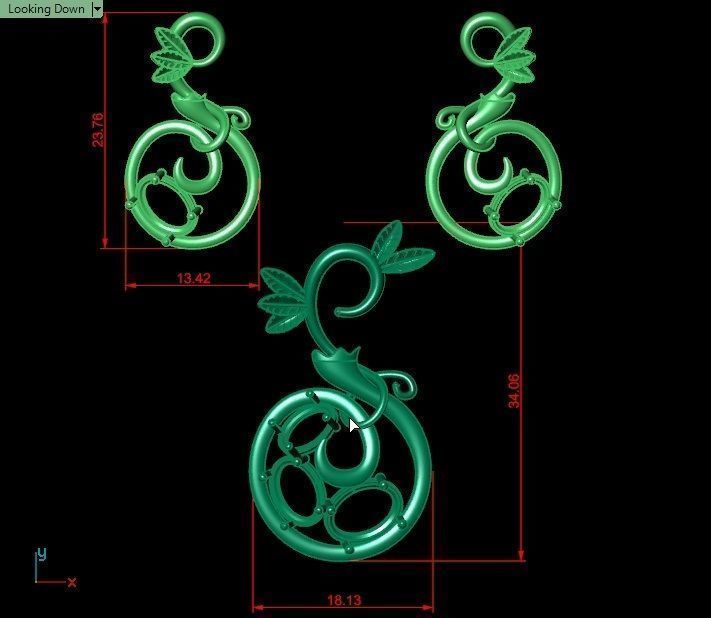
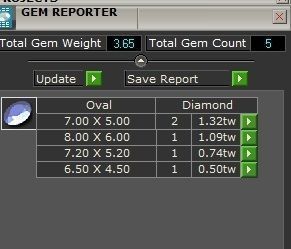
When a chromium atom replaces an occasional aluminium atom, it too loses 3 electrons to become a chromium ion to maintain the charge balance of the Al2O3 crystal. However, the Cr ions are larger and have electron orbitals in different directions than aluminium. The octahedral arrangement of the ions is distorted, and the energy levels of the different orbitals of those Cr ions are slightly altered because of the directions to the ions. Those energy differences correspond to absorption in the ultraviolet, violet, and yellow-green regions of the spectrum.